Stem cell research is a complex and dynamic field with an ever-present possibility for introduction into conservation efforts. There is a high likelihood that stem cells and targeted stem cell therapies, utilizing induced pluripotent stem cells (iSPCs), will provide treatment and possible cures for cancerous and degenerative diseases and, conserve genetic material within avian species. Although the financial aspects of stem cell research have given rise to major regulation of the field, studies conducted by Katayama, M., Fukuda, T., Kaneko, and the time-capsule team at the National Institute of Environmental Studies found novel iSPCs for three endangered species of bird in Japan which could provide an important resource for future conservation efforts.
Stem cell research can be differentiated into subcategories from where specific stem cells are derived: Induced pluripotent stem cells (iPSCs) are autologous or allogeneic material and are derived from somatic cells; Embryonic Stem Cells (ESCs) are solely allogenic and are derived from the inner cell masses of blastocyst; and Spermatogonial Stem Cells (SSCs) consist of both autologous or allogeneic material. Although ESC and SSC stem cells are credible for research, they do not have high degrees of proliferation when unisolated. iPSC cells provide the most likely candidate for conservation efforts as they are created by transforming somatic cells (which are differentiated) into pluripotent stem cells. This process is completed by forcing the expression of specific genes within a process known as somatic cell nuclear transfer. Trans-acting elements, or transcription factors, are proteins responsible for initiating transcription that bind to promoter regions of a cell. Since all cells contain the organism’s genome, differentiation (the process in which immature stem cells become matured cells with specific functions) within cells is caused by the introduction of specific transcription factors that initiate or terminate the transcription of a particular gene. Therefore, microbiologists hypothesized that if a genome were exposed to specific transcription factors, it could express the undifferentiated characteristics of a stem cell. Shinya Yamanaka created the correct conditions and provided the transcription factors necessary to induce pluripotency in somatic cells through this forced expression in 2006.
Across the globe, hundreds of avian species are endangered, and humans pose a massive threat to their survival. In Japan — where Katayama, M., Fukuda, T., Kaneko, and the time-capsule team created new iSPCs to protect three endangered bird species — 13 avian species are categorized as critically endangered (Japan - Species | CEPF, n.d.). With the preservation of biodiversity as an important goal for many conservationists, genetic preservation of threatened species using integrated stem cell technologies provides a bank of genetic information that allows differentiation into specific cellular types. Ultimately, scientific advancement in this field could save numerous avian species from extinction and help them reestablish healthy wild populations. Providing researchers with a way to access and differentiate any given bird cell allows for capabilities beyond genetic banking.
To generate the iSPCs derived from the endangered bird species in the study — the Okinawa rail (Hypotaenidia okinawae), Japanese ptarmigan (Lagopus muta japonica), and Blakiston’s fish owl (Bubo blakistoni) —, the team first exposed the cells targeted for induction (fibroblasts from the bird species that had been cryopreserved in liquid nitrogen for 8-12 years) to PB-R6F, a group of transcription factors (modified Oct3/4 (M3O), Sox2, Klf4, c-Myc, Lin28, and Nanog) known to establish iPSC’s for chickens. PB-R6F was successful for the Okinawa rail, Japanese ptarmigan, and the control subjects of chicken and mouse fibroblasts, yet it did not cause Blakiston’s fish-owl iPSC colonies to appear. To solve this problem, the team modified the polycistronic single all-in-one vector (PB-TAD-7F) to include modified Oct3/4 (M3O), Sox2, Klf4, c-Myc, Klf2, Lin28, and Nanog, resulting in the establishment of the iPSC colonies at a 5% or lower success rate using the liposuction method. A vector map of PB-TAD-7F is shown below.

With the addition of hygromycin, the experiment was repeated creating a much higher number of primary colonies of all species. The colonies' morphologies differed — the Japanese ptarmigan was three-dimensional and the Blakiston’s fish owl was flat, yet the experiment yielded Stanley passages iPSCs more than 20 times. With these iPSCs, a wide array of tests were run, including the effect of inhibitors, catalysts, and other variables.
The results of this experiment demonstrated the genetic differences between the iPSCs within the three bird species. These iPSC colonies (shown below) are the first ever established for the Okinawa rail, Japanese ptarmigan, and Blakiston’s fish owl.
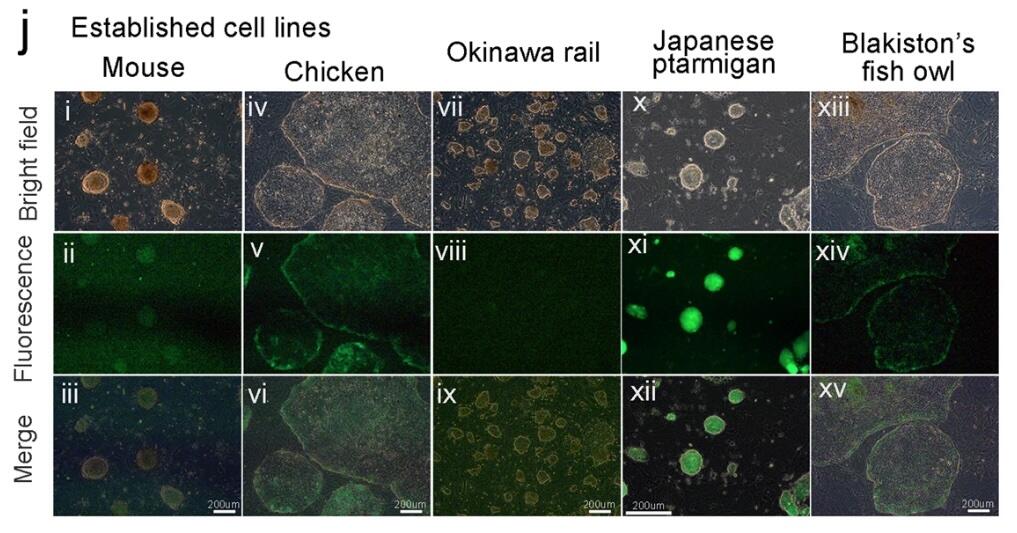
The proof that iPSC germlines can be established and differentiated within these species lays the framework for a crucial and complex way of thinking about conservation efforts. The creation of these germlines provides genetic security for endangered species, allowing for further studies to be undertaken that might preserve the health and well-being of living members of these species. In the world of human healthcare, iPSCs likewise have vast applications in transplantation medicine and nervous system repair. Research workers in 2023 developed a large-scale method for differentiating iPSCs into neural progenitor cells using additional transcription factors, Activin A, Noggin, Wnt3a, and an additional central nervous system-derived growth factor BDNF. Neural Progenitor cells show limited ability to divide and replicate within the human nervous system. Still, due to the ability to increase their potency from iPSCs, they provide therapeutic remedies to neurological diseases such as Parkinson's. Suppose this treatment could be transferred to assist in the care and preservation of endangered species. In that case, the benefits of stem cell research might well be one of humanity’s best hopes at preserving the animal kingdom.
Theoretically, just as stem cells can be differentiated into dopaminergic cells in the human brain, iPSCs could be differentiated into cells with a modified endoplasmic reticulum and/or ribosomes that could produce and transport inhibitors for common problematic chemicals, such as the nicotinamide imidacloprid (C9H10ClN5O2), with assistance from Crispr Cas9 gene modification tools. These inhibitors could either bind to the chemical or use some other form of deactivation such as catalyzing its breakdown to prevent it from causing massive damage to the organism such as changes in blood chemistry or fertility.
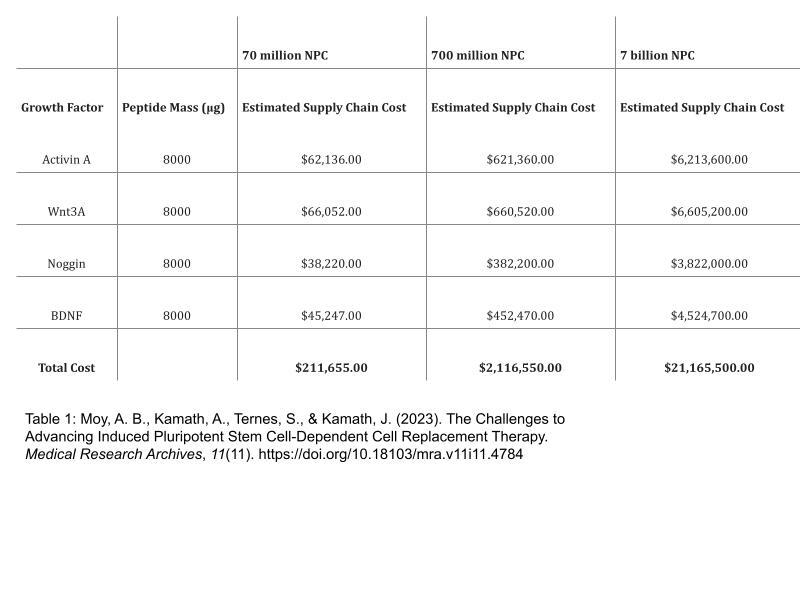
Although the steep cost of stem cell therapies is an inhibiting factor, an outpouring of research towards human treatment of neurological diseases using iPSCs has significantly lowered costs. A dose of 4.8 million dopaminergic neural progenitor cells (NPC’s) derived from the large-scale differentiation process would provide a large enough dose for treatment. A total of 70 million cells would result in sufficient dosage for 14 subjects for clinical trials and modern mass production of these cells has little to no chance for mutation. The cost of the growth factors required for this treatment is minimal due to mass production. Conservationists could piggyback off the massive amount of human-oriented research in stem cell therapies and transfer it to creating growth factors to differentiate avian cells.
Currently, there is little to no usage of iPSCs in remedial care for endangered birds, but developments within the field and the proof of plausibility for stem cell germlines to be established within specific bird species give hope for a new era of animal conservation. NOAA provides insights into the importance of maintaining genetic banks within critically endangered species. Their research supports maintaining genetic diversity and the ability to conserve stem cells, which have differentiation capabilities, provide a form of resilience to small or isolated populations, and help these species adapt to future environmental changes with assistance from iPSC technologies.
As of now, genetic conservation using iPSCs is a novel form of environmental conservation, yet is a promising branch of environmentalism as costs decrease and capabilities skyrocket. With shrinking habitats and warming seas along with microplastics and man-made chemicals destroying environments, it is of tremendous importance that searching for new ways of species conservation is a continuity in the scientific world to help preserve biodiversity for generations to come.
Bibliography
Anderson, Meredith J et al. “Imidacloprid exposure is detectable in over one third of wild bird samples from diverse Texas ecoregions.” The Science of the Total Environment vol. 876 (2023): 162723. doi:10.1016/j.scitotenv.2023.162723
Blackledge, Steve (2024, April 24). EPA Report says pesticides endanger wildlife Environment America. Retrieved August 14, 2024, from https://environmentamerica.org/articles/epa-report-says-pesticides-endanger-wildlife/
Fisheries, N. (2023, February 21). Preserving Genetic Diversity Gives Wild Populations Their Best Chance at Long-Term Survival. NOAA. Retrieved August 14, 2024, from https://www.fisheries.noaa.gov/feature-story/preserving-genetic-diversity-gives-wild-populations-their-best-chance-long-term#:~:text=Maintaining%20high%20genetic%20diversity%20allows,ability%20to%20survive%20and%20reproduce.
Herberts, C. A., Kwa, M. S., & Hermsen, H. P. (2011). Risk factors in the development of stem cell therapy. Journal of Translational Medicine, 9(1). https://doi.org/10.1186/1479-5876-9-29
Hinoi, E., Balcar, V. J., Kuramoto, N., Nakamichi, N., & Yoneda, Y. (2002). Nuclear transcription factors in the hippocampus. Progress in Neurobiology, 68(2), 145–165. https://doi.org/10.1016/s0301-0082(02)00078-3
Japan - Species | CEPF. (n.d.). https://www.cepf.net/our-work/biodiversity-hotspots/japan/species
Jentoft, I. M. A., Bäuerlein, F. J. B., Welp, L. M., Cooper, B. H., Petrovic, A., So, C., Penir, S. M., Politi, A. Z., Horokhovskyi, Y., Takala, I., Eckel, H., Moltrecht, R., Lénárt, P., Cavazza, T., Liepe, J., Brose, N., Urlaub, H., Fernández-Busnadiego, R., & Schuh, M. (2023). Mammalian oocytes store proteins for the early embryo on cytoplasmic lattices. Cell, 186(24), 5308–5327.e25. https://doi.org/10.1016/j.cell.2023.10.003
Katayama, M., Fukuda, T., Kaneko, T. et al. Induced pluripotent stem cells of endangered avian species. Commun Biol 5, 1049 (2022). https://doi.org/10.1038/s42003-022-03964-y
Martínez, P., & Blasco, M. A. (2017). Telomere-driven diseases and telomere-targeting therapies. the Journal of Cell Biology/the Journal of Cell Biology, 216(4), 875–887. https://doi.org/10.1083/jcb.201610111
Moy, A. B., Kamath, A., Ternes, S., & Kamath, J. (2023). The Challenges to Advancing Induced Pluripotent Stem Cell-Dependent Cell Replacement Therapy. Medical research archives, 11(11), 4784. https://doi.org/10.18103/mra.v11i11.4784
National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 86287518, Imidacloprid. Retrieved August 14, 2024 from https://pubchem.ncbi.nlm.nih.gov/compound/Imidacloprid.
Noisa, P., Raivio, T., & Cui, W. (2015). Neural Progenitor Cells Derived from Human Embryonic Stem Cells as an Origin of Dopaminergic Neurons. Stem Cells International, 2015, 1–10. https://doi.org/10.1155/2015/647437
Okita, K., Ichisaka, T., & Yamanaka, S. (2007). Generation of germline-competent induced pluripotent stem cells. Nature, 448(7151), 313–317. https://doi.org/10.1038/nature05934
Omole, A. E., & Fakoya, A. O. J. (2018). Ten years of progress and promise of induced pluripotent stem cells: historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ, 6, e4370. https://doi.org/10.7717/peerj.4370
Powell, H. (2022, August 31). Neonic Nation: Is Widespread Pesticide Use Connected to Grassland Bird Declines? All About Birds. https://www.allaboutbirds.org/news/neonic-nation-is-widespread-pesticide-use-connected-to-grassland-bird-declines/
“Stem cells: What they are and what they do.” Mayo Clinic, 23 Mar. 2024, www.mayoclinic.org/tests-procedures/bone-marrow-transplant/in-depth/stem-cells/art-20048117#:~:text=Stem%20cells%20are%20a%20special,all%20tissues%20of%20the%20body.
Threats to Birds | U.S. Fish & Wildlife Service. (n.d.). FWS.gov. https://www.fws.gov/library/collections/threats-birds
Ye, L., Swingen, C., & Zhang, J. (2013). Induced pluripotent stem cells and their potential for basic and clinical sciences. Current cardiology reviews, 9(1), 63–72. https://doi.org/10.2174/157340313805076278
Yu, J., Vodyanik, M. A., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J. L., Tian, S., Nie, J., Jonsdottir, G. A., Ruotti, V., Stewart, R., Slukvin, I. I., & Thomson, J. A. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science (New York, N.Y.), 318(5858), 1917–1920. https://doi.org/10.1126/science.1151526
Zhu, L. J. (2015). Overview of guide RNA design tools for CRISPR-Cas9 genome editing technology. Frontiers in Biology, 10(4), 289–296. https://doi.org/10.1007/s11515-015-1366-y


